publications
Here is a selected list of some of my published works.
2025
-
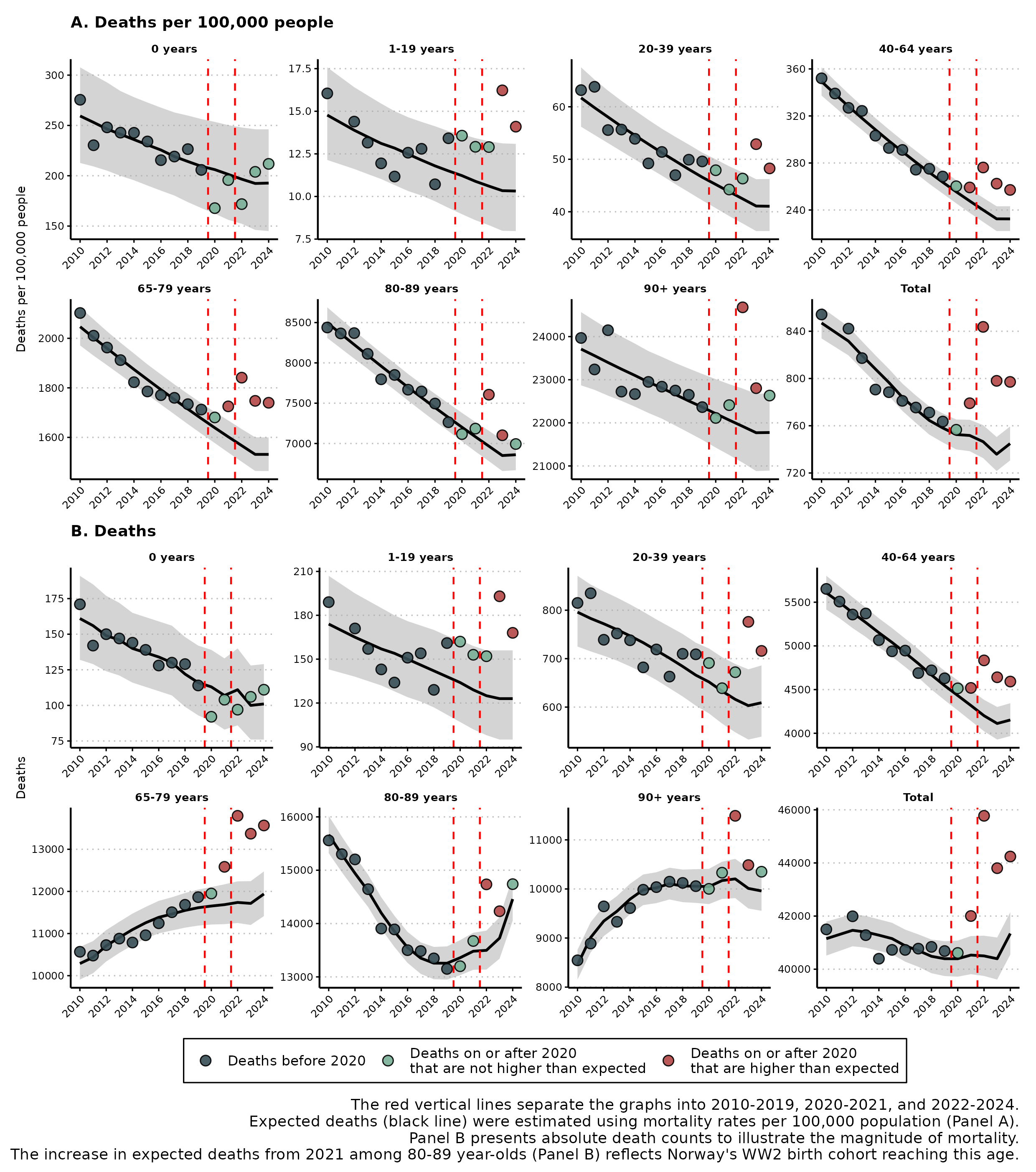 Excess all-cause mortality in Norway in 2024Richard Aubrey White , Anders B. Nygaard , Arne Søraas , and 1 more authorScandinavian Journal of Public Health, Sep 2025Publisher: SAGE Publications Ltd STM
Excess all-cause mortality in Norway in 2024Richard Aubrey White , Anders B. Nygaard , Arne Søraas , and 1 more authorScandinavian Journal of Public Health, Sep 2025Publisher: SAGE Publications Ltd STMAims: The Norwegian Institute of Public Health calculated excess mortality for Norway in 2024 using a reference period that included 2023—a year with significant excess mortality—and concluded there was no excess mortality in 2024. This study estimates excess mortality in 2024 using only pre-pandemic years as the reference, providing a basis for identifying excess COVID-19 related mortality. Methods: We estimated excess mortality in 2024 using a negative binomial model trained on 2010–2019 data. Deaths were modelled by age (0, 1–19, 20–39, 40–64, 65–79, 80–89 and 90+ years) and sex, with population offsets. Expected mortality was projected using both a conservative approach where the prediction for 2023 was carried forward to 2024 and a non-conservative linear extrapolation to 2024. Results: The conservative approach estimated 2898 excess deaths (7.0%; 95% prediction interval (PI), 4.9–9.1%) in 2024. Significant excess mortality was observed in age groups 1–19 (45 deaths; 36.6% excess), 20–39 (107 deaths; 17.6% excess), 40–64 (439 deaths; 10.6% excess) and 65–79 (1631 deaths; 13.7% excess). Ages 1–39 and 40–64 accounted for approximately 5% and 15% of total excess mortality, respectively. Conclusions: Persistent excess mortality from 2022 to 2024 suggests a new elevated mortality baseline and a reduction or reversal of Norway’s pre-pandemic mortality decline. Although multiple factors may contribute, given sustained excess mortality since 2022, our findings suggest that the unmitigated spread of SARS-CoV-2 in Norway since 2022 can be associated with increased mortality, particularly for those under 65.
2024
-
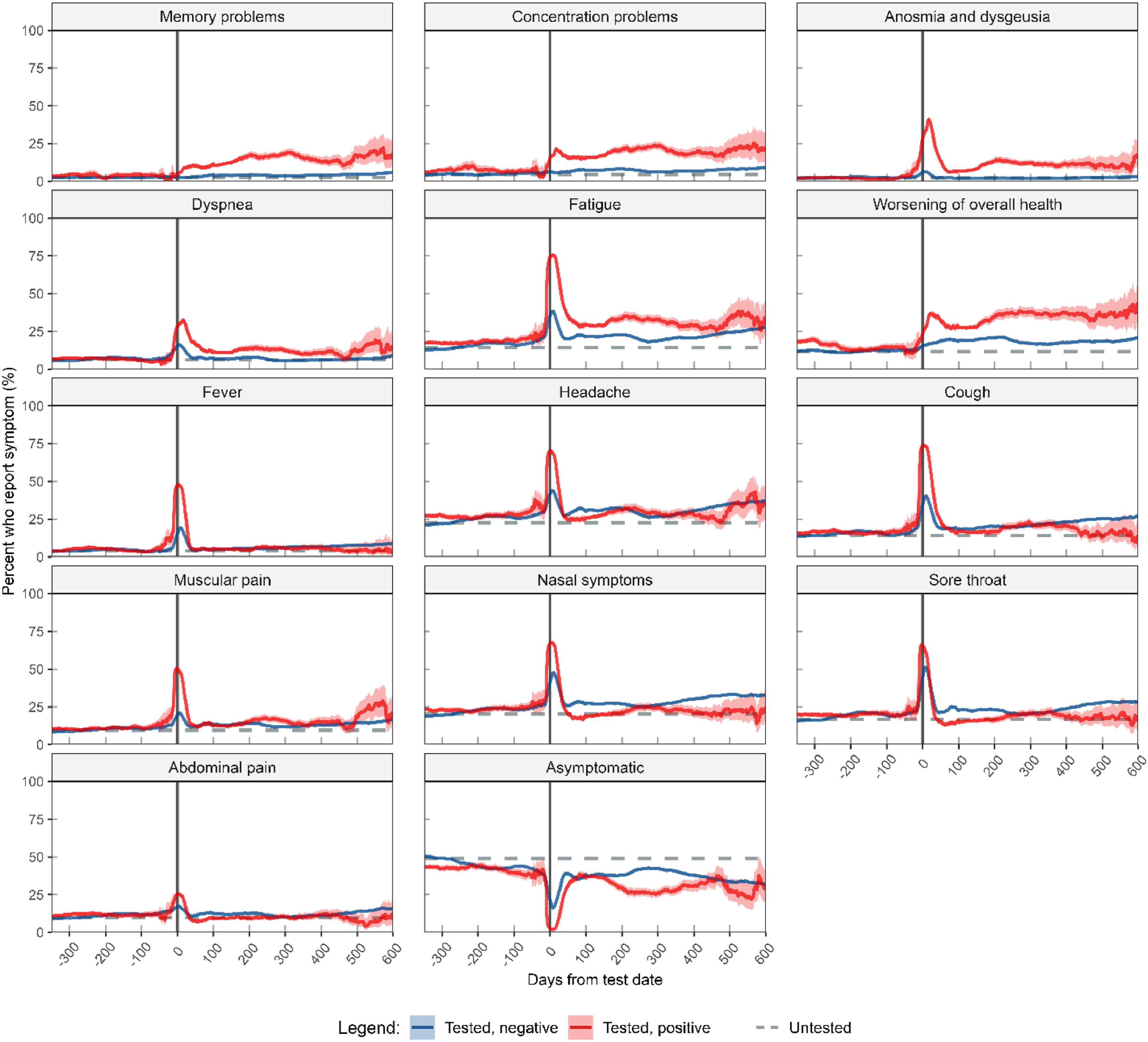 Temporal trajectories of long-COVID symptoms in adults with 22 months follow-up in a prospective cohort study in NorwayMerete Ellingjord-Dale , Anders Benteson Nygaard , Nathalie C. Støer , and 11 more authorsInternational Journal of Infectious Diseases, Oct 2024Authorship: Shared first author
Temporal trajectories of long-COVID symptoms in adults with 22 months follow-up in a prospective cohort study in NorwayMerete Ellingjord-Dale , Anders Benteson Nygaard , Nathalie C. Støer , and 11 more authorsInternational Journal of Infectious Diseases, Oct 2024Authorship: Shared first authorObjectives: There is a lack of large studies on long-COVID symptoms with symptoms measurements before the onset of COVID-19. Therefore, long-COVID is still poorly defined. Methods: The Norwegian COVID-19 Cohort Study is a population-based, open cohort of adult participants (aged 18-96 years) from Norway. From March 27, 2020, participants were recruited through social media, invitations, and nationwide media coverage. Fourteen somatic and cognitive symptoms were assessed at baseline and four follow-ups for up to 22 months. SARS-CoV-2 test status was obtained from a mandatory national registry or from self-report. Results: After follow-up, 15 737 participants had a SARS-CoV-2-positive test, 67 305 had a negative test, and 37 563 were still untested. Persistent symptoms reported more frequently by positive compared with negative participants one month after infection, were memory problems (3-6 months: adjusted odds ratio (aOR) = 6.8, CI = 5.7-8.1; >18 months: aOR = 9.4, CI = 4.1-22), and concentration problems (3-6 months: aOR = 4.1, CI = 3.5-4.7; >18 months: aOR = 4.4, CI = 2.0-9.7) as well fatigue, dyspnea, anosmia and dysgeusia. Conclusions: COVID-19 was associated with cognitive symptoms, anosmia, dysgeusia, dyspnea, and fatigue as well as worsening of overall health up to 22 months after a SARS-CoV-2 test, even when correcting for symptoms before the onset of COVID-19.
-
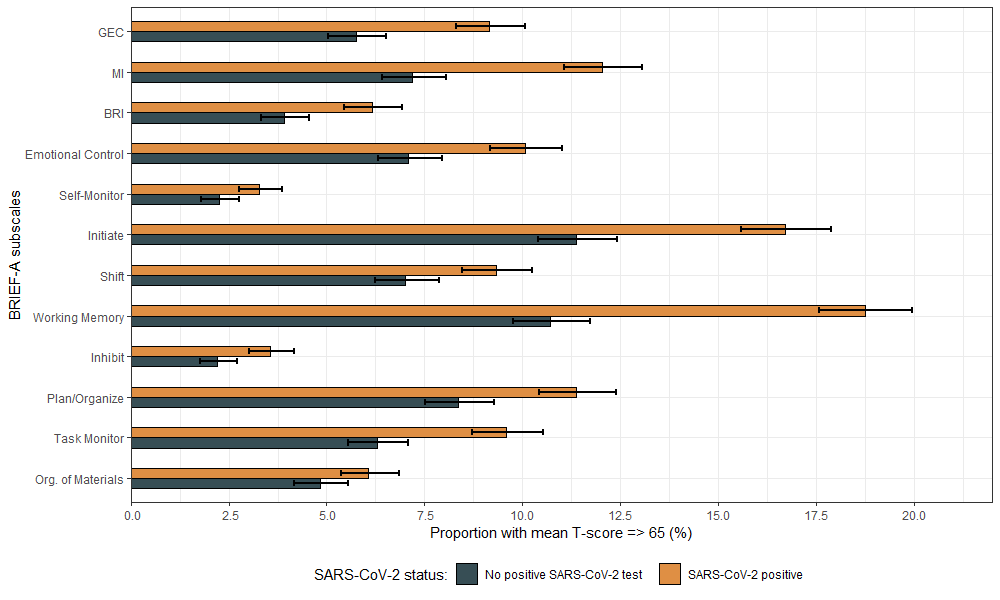 Executive deficits after SARS-CoV-2 infection: A cross-sectional population studyS. Buer , B. I. Hagen , A. Søraas , and 9 more authorsBrain, Behavior, & Immunity - Health, Nov 2024Authorship: Last author
Executive deficits after SARS-CoV-2 infection: A cross-sectional population studyS. Buer , B. I. Hagen , A. Søraas , and 9 more authorsBrain, Behavior, & Immunity - Health, Nov 2024Authorship: Last authorImportance: Despite the major implications of executive deficits in day-to-day functioning, few studies have investigated this in post-acute sequelae of SARS-CoV-2 infection using standardized measures that differentiate between aspects of executive function. Objective: Examine whether SARS-CoV-2 infection is associated with deficits in executive functions and if so, investigate the duration of this association. Design, Setting, and Participants: The present research has a cross-sectional design and uses data from the Norwegian Covid-19 Cohort study. The current cohort (n = 8102) completed the Behavior Rating Inventory of Executive Function- Adult Version (BRIEF-A) electronically between April 2021 and September 2021. During the assessment, 4183 of the included participants had a prior positive polymerase chain reaction test (PCR) for SARS-CoV-2 and 3919 were untested or had a confirmed negative PCR test. Exposure: Laboratory-confirmed SARS-CoV-2 infection. Main outcomes and measures: Executive functions were measured using the BRIEF-A, a self-report questionnaire comprising 75 items within nine theoretically and empirically distinct clinical scales. All participants self-reported on demographical variables and comorbidity. Information on sex and age was derived from the personal identification number, and vaccination status was obtained from the Norwegian Immunization Registry (SYSVAK). Results: Participants with a positive SARS-CoV-2 status reported executive deficits in everyday life above the clinical threshold (T-score ≥65) more often than non-infected controls (383 vs. 225). Specifically, the SARS-CoV-2 positive status group indicated significantly more deficits related to metacognition, with the greatest difference demonstrated for working memory. This difference remained when adjusting for various demographic factors and comorbidities, with significantly greater odds of reporting above the clinical threshold following SARS-CoV-2 infection, as observed on the global executive composite score 6–12 months after infection (OR 1.97; 95% CI 1.51 to 2.55). Conclusions: Our study confirms more perceived executive deficits following SARS-CoV-2 infection compared to non-infected controls, with metacognitive aspects being the most affected. These findings shed light on the potential functional difficulties that individuals may encounter during the post-acute phase of SARS-CoV-2 infection and may guide further development of targeted interventions addressing metacognitive domains of executive functioning.
- Post-COVID-19 condition symptoms 6–12 months after hospitalisation in WuhanArne Søraas , Sonja H. Brunvoll , Anders B. Nygaard , and 2 more authorsThe Lancet, Apr 2024
2023
- Post-acute symptoms 3-15 months after COVID-19 among unvaccinated and vaccinated individuals with a breakthrough infectionSonja H. Brunvoll , Anders B. Nygaard , Morten W. Fagerland , and 4 more authorsInternational Journal of Infectious Diseases, Jan 2023Authorship: Shared first author
Objective: We aimed to describe post-acute sequelae of SARS-CoV-2 infection (PASC) related symptoms 3-15 months after a positive test in SARS-CoV-2 unvaccinated and vaccinated participants with a breakthrough infection. Methods: Participants of the Norwegian COVID-19 cohort, without a positive SARS-CoV-2 test, completed a questionnaire about PASC-related symptoms between November 2020 and January 2021. About a year later, a second questionnaire (which also included the Everyday Memory Questionnaire [EMQ]-13) was completed by the same participants, most still without a positive SARS-CoV-2 test, but also by unvaccinated and vaccinated participants with a positive test 3-15 months before the questionnaire. Laboratory-confirmed SARS-CoV-2 status (positive or negative swab test determined by reverse transcriptase quantitative polymerase chain reaction) at the time of completing the questionnaire was ascertained from the Mandatory Norwegian Surveillance System for Communicable Diseases. Results: No differences were found in the self-reported PASC symptoms, dyspnea, fatigue, smell/taste changes, concentration problems, or the EMQ-13 score between unvaccinated and vaccinated participants 3-15 months after the positive test. Fewer memory problems were reported among vaccinated than unvaccinated participants. Conclusion: SARS-CoV-2 vaccines offer minor protection against PASC symptoms, although fewer memory problems were reported among the vaccinated than the unvaccinated participants.
2022
-
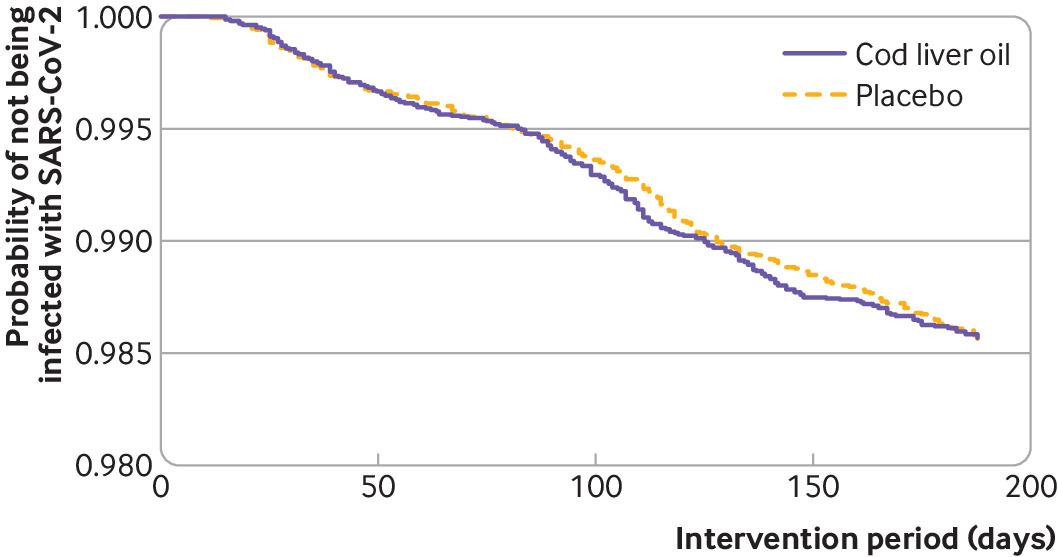 Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trialSonja H. Brunvoll , Anders B. Nygaard , Merete Ellingjord-Dale , and 12 more authorsBMJ, Sep 2022Authorship: Shared first author
Prevention of covid-19 and other acute respiratory infections with cod liver oil supplementation, a low dose vitamin D supplement: quadruple blinded, randomised placebo controlled trialSonja H. Brunvoll , Anders B. Nygaard , Merete Ellingjord-Dale , and 12 more authorsBMJ, Sep 2022Authorship: Shared first authorObjective: To determine if daily supplementation with cod liver oil, a low dose vitamin D supplement, in winter, prevents SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections in adults in Norway. Design: Quadruple blinded, randomised placebo controlled trial. Setting: Norway, 10 November 2020 to 2 June 2021. Participants: 34 601 adults (aged 18-75 years), not taking daily vitamin D supplements. Intervention: 5 mL/day of cod liver oil (10 µg of vitamin D, n=17 278) or placebo (n=17 323) for up to six months. Main outcome measures: Four co-primary endpoints were predefined: the first was a positive SARS-CoV-2 test result determined by reverse transcriptase-quantitative polymerase chain reaction and the second was serious covid-19, defined as self-reported dyspnoea, admission to hospital, or death. Other acute respiratory infections were indicated by the third and fourth co-primary endpoints: a negative SARS-CoV-2 test result and self-reported symptoms. Side effects related to the supplementation were self-reported. The fallback method was used to handle multiple comparisons. Results: Supplementation with cod liver oil was not associated with a reduced risk of any of the co-primary endpoints. Participants took the supplement (cod liver oil or placebo) for a median of 164 days, and 227 (1.31%) participants in the cod liver oil group and 228 (1.32%) participants in the placebo group had a positive SARS-CoV-2 test result (relative risk 1.00, multiple comparison adjusted confidence interval 0.82 to 1.22). Serious covid-19 was identified in 121 (0.70%) participants in the cod liver oil group and in 101 (0.58%) participants in the placebo group (1.20, 0.87 to 1.65). 8546 (49.46%) and 8565 (49.44%) participants in the cod liver oil and placebo groups, respectively, had ≥1 negative SARS-CoV-2 test results (1.00, 0.97 to 1.04). 3964 (22.94%) and 3834 (22.13%) participants in the cod liver oil and placebo groups, respectively, reported ≥1 acute respiratory infections (1.04, 0.97 to 1.11). Only low grade side effects were reported in the cod liver oil and placebo groups. Conclusion: Supplementation with cod liver oil in the winter did not reduce the incidence of SARS-CoV-2 infection, serious covid-19, or other acute respiratory infections compared with placebo. Trial registration: ClinicalTrials.gov NCT04609423.
-
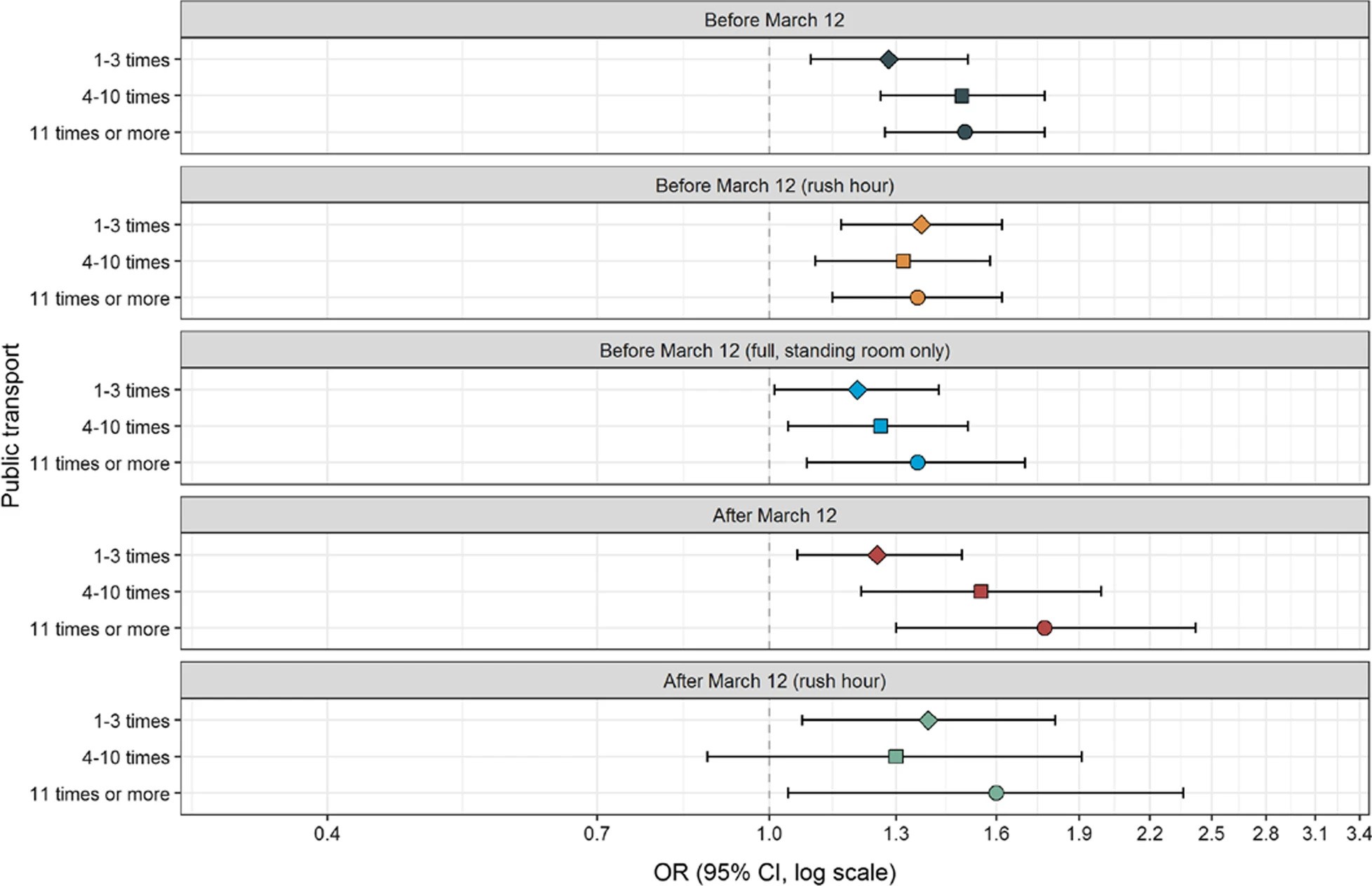 The use of public transport and contraction of SARS-CoV-2 in a large prospective cohort in NorwayMerete Ellingjord-Dale , Karl Trygve Kalleberg , Mette S. Istre , and 7 more authorsBMC Infectious Diseases, Mar 2022
The use of public transport and contraction of SARS-CoV-2 in a large prospective cohort in NorwayMerete Ellingjord-Dale , Karl Trygve Kalleberg , Mette S. Istre , and 7 more authorsBMC Infectious Diseases, Mar 2022For many people public transport is the only mode of travel, and it can be challenging to keep the necessary distances in such a restricted space. The exact role of public transportation and risk of SARS-CoV-2 transmission is not known.
- ISARIC-COVID-19 dataset: A Prospective, Standardized, Global Dataset of Patients Hospitalized with COVID-19Esteban Garcia-Gallo , Laura Merson , Kalynn Kennon , and 23 more authorsSci Data, Jul 2022
The International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) COVID-19 dataset is one of the largest international databases of prospectively collected clinical data on people hospitalized with COVID-19. This dataset was compiled during the COVID-19 pandemic by a network of hospitals that collect data using the ISARIC-World Health Organization Clinical Characterization Protocol and data tools. The database includes data from more than 705,000 patients, collected in more than 60 countries and 1,500 centres worldwide. Patient data are available from acute hospital admissions with COVID-19 and outpatient follow-ups. The data include signs and symptoms, pre-existing comorbidities, vital signs, chronic and acute treatments, complications, dates of hospitalization and discharge, mortality, viral strains, vaccination status, and other data. Here, we present the dataset characteristics, explain its architecture and how to gain access, and provide tools to facilitate its use.
- Breakthrough infections with the omicron and delta variants of SARS-CoV-2 result in similar re-activation of vaccine-induced immunityArne Søraas , Gunnveig Grødeland , Beathe Kiland Granerud , and 29 more authorsFrontiers in Immunology, Jul 2022
BackgroundResults showing that sera from double vaccinated individuals have minimal neutralizing activity against Omicron have been interpreted as indicating the need for a third vaccine dose for protection. However, there is little information about early immune responses to Omicron infection in double vaccinated individuals.MethodsWe measured inflammatory mediators, antibodies to the SARS-CoV-2 spike and nucleocapsid proteins, and spike peptide-induced release of interferon gamma in whole blood in 51 double-vaccinated individuals infected with Omicron, in 14 infected with Delta, and in 18 healthy controls. The median time points for the first and second samples were 7 and 14 days after symptom onset, respectively.FindingsInfection with Omicron or Delta led to a rapid and similar increase in antibodies to the receptor-binding domain (RBD) of Omicron protein and spike peptide-induced interferon gamma in whole blood. Both the Omicron- and the Delta-infected patients had a mild and transient increase in inflammatory parameters.InterpretationThe results suggest that two vaccine doses are sufficient to mount a rapid and potent immune response upon infection in healthy individuals of with the Omicron variant.FundingThe study was funded by the Oslo University Hospital, and by grants from The Coalition for Epidemic Preparedness Innovations, Research Council of Norway (no 312780, 324272), South-Eastern Norway Regional Health Authority (no 2019067, 2021071, 10357, 2021047, 33612, 2021087, 2017092), EU Horizon 2020 grant no 848099, a philantropic donation from Vivaldi Invest A/S, and The European Virus Archive Global.
- Symptom‐based case definitions for COVID‐19: Time and geographical variations for detection at hospital admission among 260,000 patientsJoaquin Baruch , Amanda Rojek , Christiana Kartsonaki , and 7 more authorsInfluenza and Other Respiratory Viruses, Jul 2022
2020
-
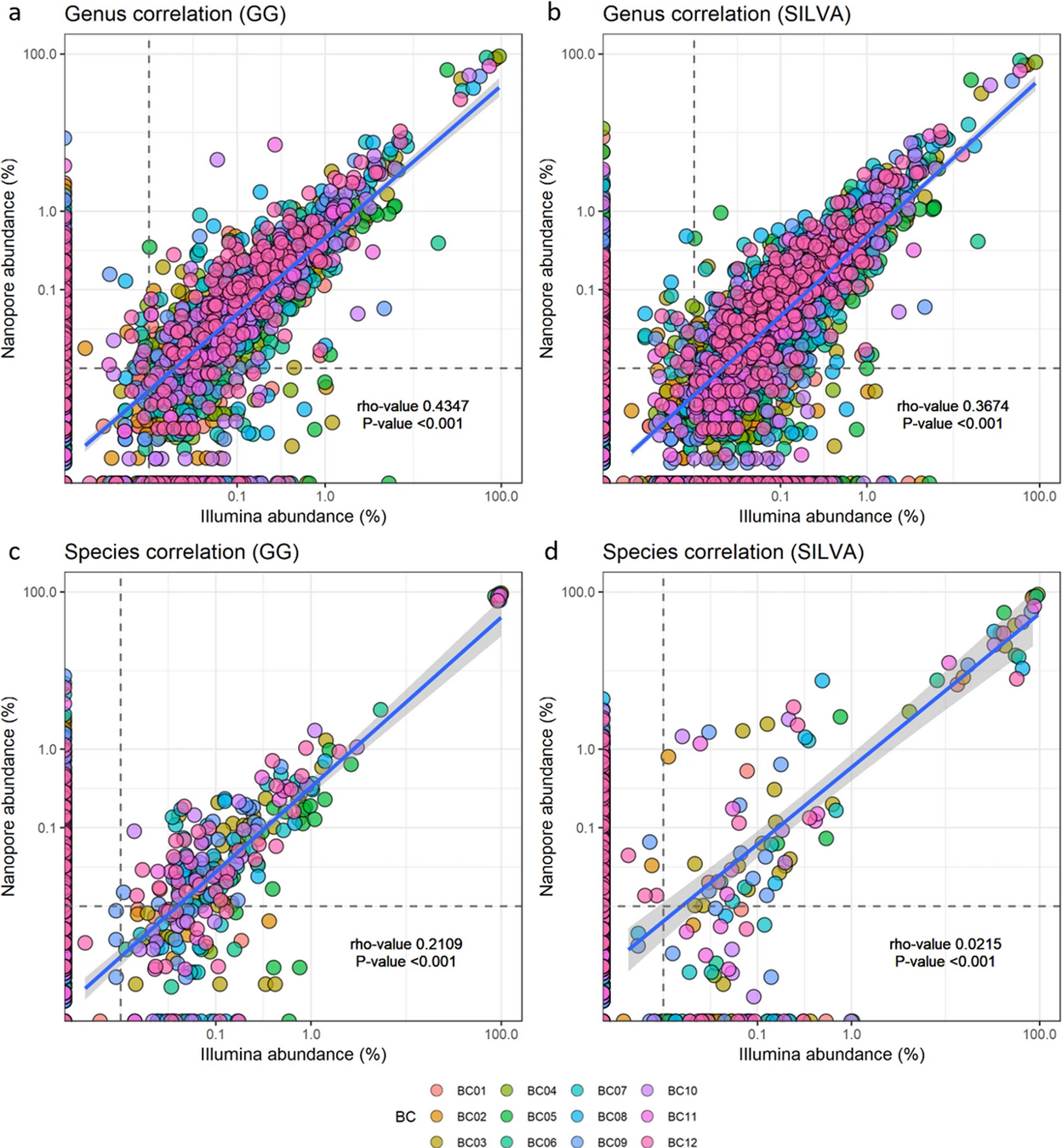 A preliminary study on the potential of Nanopore MinION and Illumina MiSeq 16S rRNA gene sequencing to characterize building-dust microbiomesAnders B. Nygaard , Hege S. Tunsjø , Roger Meisal , and 1 more authorScientific Reports, Dec 2020Authorship: First author
A preliminary study on the potential of Nanopore MinION and Illumina MiSeq 16S rRNA gene sequencing to characterize building-dust microbiomesAnders B. Nygaard , Hege S. Tunsjø , Roger Meisal , and 1 more authorScientific Reports, Dec 2020Authorship: First authorAbstract There is a growing awareness of the importance of indoor microbiomes for human health. Given their complexity, these microbiomes can only be adequately surveyed using high throughput sequencing techniques. Oxford Nanopore’s MinION is the newest third generation sequencing technology on the market. With its many advantages such as portability, user friendliness, simplicity, speed of sequencing and long read length, the technology is now an actual contender to established sequencing platforms. MinION’s main disadvantage is a relatively low read accuracy compared to several other platforms, although this is constantly improving. The present study, which appears to be the first of its kind, provides the results of a preliminary analysis of the microbial communities in indoor environments based on 16S rRNA gene amplicon sequencing, using both the Oxford Nanopore Technologies (ONT) MinIOn and the Illumina MiSeq DNA sequencers. At the level of family and above, there was no significant difference between the microbial compositions as revealed by the two platforms. However, at the genus, and particularly at the species level, the ONT MinION reported greater taxonomic resolution than Illumina MiSeq.
- Whole genome sequencing and antibiotic diffusion assays, provide new insight on drug resistance in the genus PedobacterIngvild F. Ullmann , Anders B. Nygaard , Hege S. Tunsjø , and 1 more authorFEMS Microbiology Ecology, Jun 2020
A total of four strains of the ‘environmental superbug’ Pedobacter isolated from sludge produced at Norwegian drinking water treatment plants, were characterized by whole genome sequencing and antibiotic susceptibility assays. As with previous studies on members of this genus, we found that the isolates were multi-drug resistant, and that this resistance included clinically important beta-lactams, aminoglycosides and the fluoroquinolone ciprofloxacin. Using the minION sequencing platform (Oxford Nanopore Technologies) combined with HiSeq PE150 Illumina sequencing data, the four isolates were assembled into genomes of single contigs. Analysis of the genomes revealed potential genetic factors possibly underlying some of the specific resistances observed. Metallo-beta-lactamase activity was detected in one isolate, and the same isolate contained a putative metallo-betalactamase gene resembling pedo-2. Furthermore, several genes related to multidrug efflux systems were found using the resistance database CARD. Additionally, the present study extends our knowledge on the phylogeny of this genus, adding four new genomes to the existing 50.
2018
-
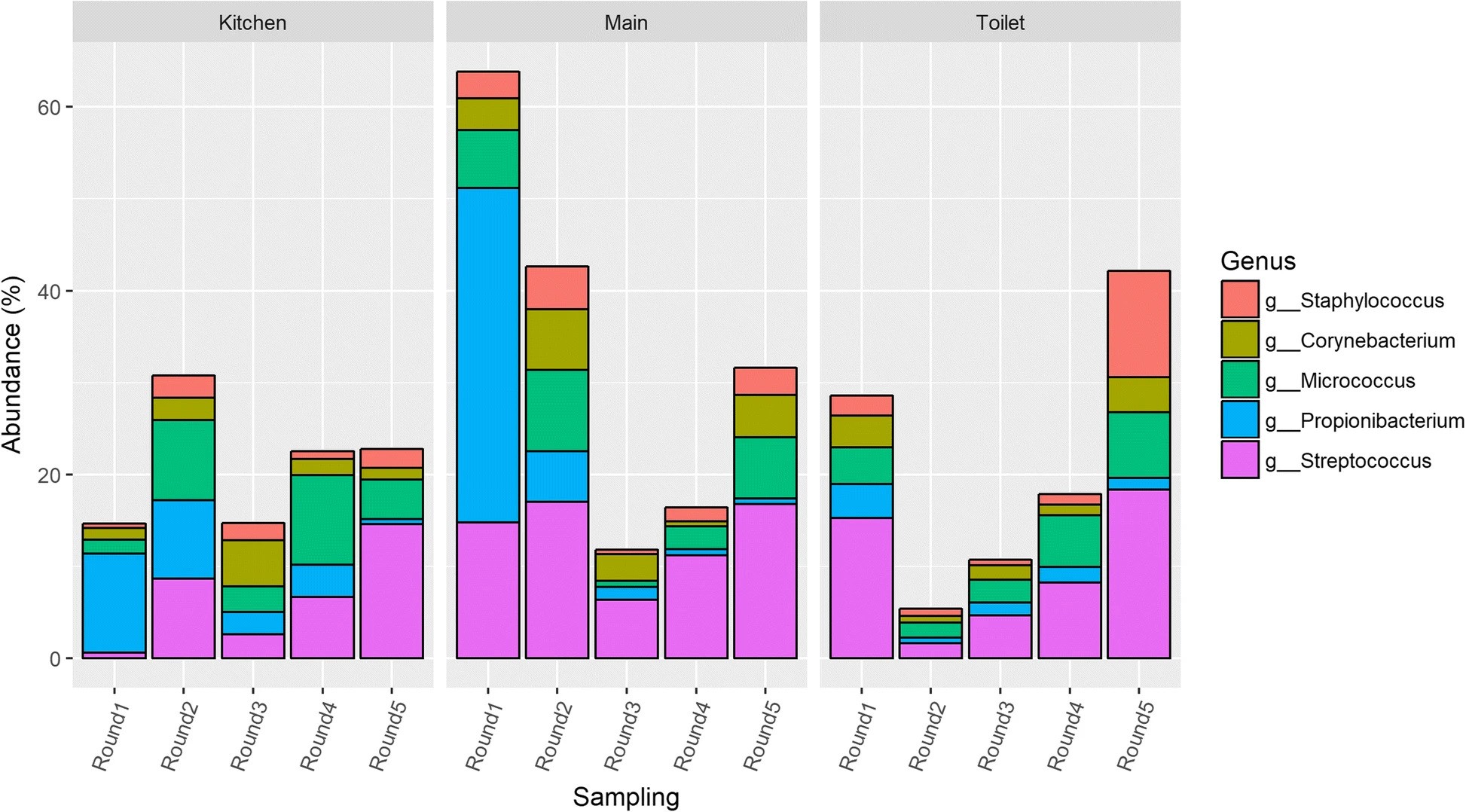 Longitudinal development of the dust microbiome in a newly opened Norwegian kindergartenAnders B. Nygaard , and Colin CharnockMicrobiome, Sep 2018Authorship: First author
Longitudinal development of the dust microbiome in a newly opened Norwegian kindergartenAnders B. Nygaard , and Colin CharnockMicrobiome, Sep 2018Authorship: First authorIn Norway, 91% of children aged 1–5 attend kindergarten where they are exposed to indoor microbiomes which can have relevance for development and health. In order to gain a better understanding of the composition of the indoor microbiome and how it is affected by occupancy over time, floor dust samples from a newly opened kindergarten were investigated. Samples were collected during an 11-month period. Samples were analyzed for bacterial composition using 16S rRNA gene sequencing. Samples were also screened for four clinically relevant antibiotic resistance genes. In addition, Petrifilm analyses were used to evaluate surface hygiene.
- The bacterial composition of ventilation filter dust in Norwegian pre-school nurseriesAnders B. Nygaard , and Colin CharnockIndoor and Built Environment, Dec 2018Authorship: First author
The microbial content of dust collected from intake and exhaust air filters in six Norwegian nurseries was determined using 16S rRNA pyrosequencing and plate count analyses. The concentration of endotoxins was also estimated. About 96% of the sequences were classified as Actinobacteria, Proteobacteria, Firmicutes, Bacteroidetes and Cyanobacteria. At the genus level, about 30% of sequences from the exhaust filter were classified as bacteria of probable human origin, such as Streptococcus and Corynebacterium species. These were close to absent in intake dust samples (\textless1%). This suggests that occupancy shapes the indoor microbiota, creating an environment relatively rich in genera of potential health significance. There were significantly greater counts of culturable bacteria and fungi in exhaust samples, indicating that passage of air through the nursery causes deterioration in the general air quality. Although there was more endotoxin in exhaust dust, the endotoxin levels per colony forming unit were similar in both samples. This study explores, for the first time, the bacterial composition of ventilation filter dust in Norwegian nurseries, and is important as it reveals what types of microorganisms nursery users are exposed to. In addition to possible direct health issues, the nature of our early exposure to microbes may be significant in the development of immunological conditions.